Caesium Atomic Number
Caesium, also spelt as Cesium, was discovered in 1860. It is outstanding in keeping time with precision, so it is used in atomic clocks. It forms alloys with alkali metals, gold and mercury.
History and Discovery
Atomic Number of Caesium Atomic Number of Caesium is 55. Chemical symbol for Caesium is Cs. Number of protons in Caesium is 55.

Cesium or caesium is a metal with the element symbol Cs and atomic number 55. This chemical element is distinctive for several reasons. Here is a collection of cesium element facts and atomic data. Name: Cesium Symbol: Cs Atomic Number: 55 Atomic Mass: 132.90546 amu Melting Point: 28.5 °C (301.65 K, 83.3 °F) Boiling Point: 678.4 °C (951.55005 K, 1253.12 °F) Number of Protons/Electrons: 55 Number of Neutrons: 78 Classification: Alkali Metal Crystal Structure: Cubic Density @ 293 K: 1.873 g/cm 3 Color: silver British Spelling: Caesium IUPAC Spelling: Caesium.
Caesium is considered the first element who was discovered spectroscopically in 1860 by Robert Bunsen and Gustav Kirchhoff. They also derived its name from Latin caesius ‘’sky-blue’’ due to the formation of unique blue lines in the emission spectrum. [1]
Cesium
| Periodic Table Classification | Group 1 Period 6 |
|---|---|
| State at 20C | Solid |
| Color | Pale gold |
| Electron Configuration | [Xe] 6s1 |
| Electron Number | 55 |
| Proton Number | 55 |
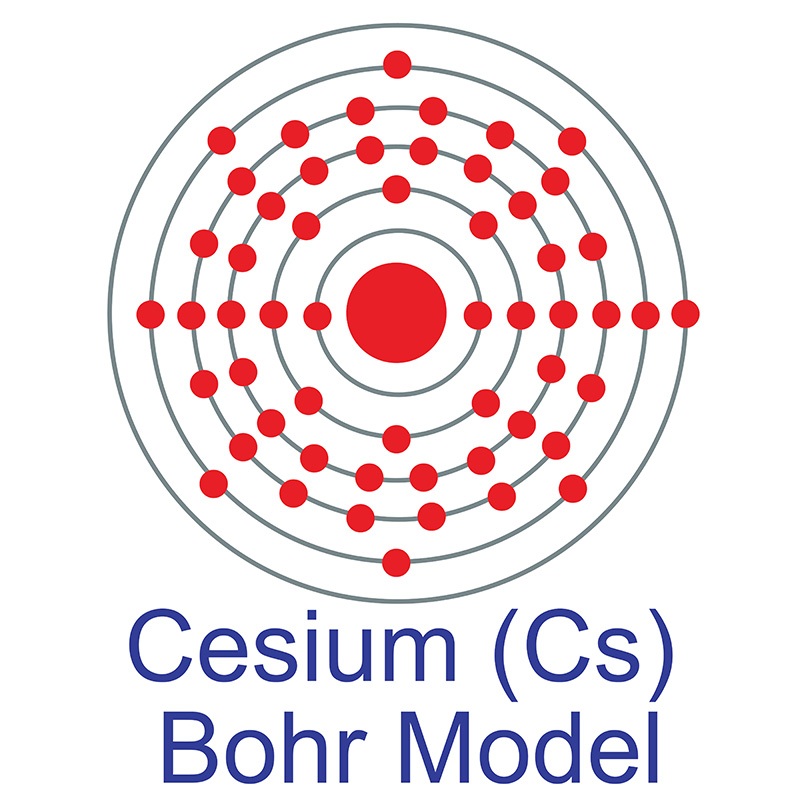
| Electron Shell | 2, 8, 18, 18, 8, 1 |
| Density | 1.87 g.cm-3 at 20°C |
| Atomic number | 55 |
| Atomic Mass | 132.91 g.mol -1 |
| Electronegativity according to Pauling | 0.79 |
Occurrence
Caesium occurs in minute quantity in earth crust in the form of minerals like pollucite (zeolite mineral Caesium ore). It is mostly present with rubidium in nature and other alkali metals. The abundance of Caesium on the earth crust is about 3 part per million and it is the 50th most common element in the earth crust [2].
Physical Characteristics
Caesium is a silvery-gold metallic element. It is an extremely soft metal. Caesium is one of four elements who exist in liquid form at or near room temperature. In periodic table it belongs to alkaline elements. Its physical properties are similar with rubidium and potassium. In the presence of mineral oil it loses its metallic luster. It has the melting point of 28.5oC and also has a low boiling point 641oC. Its golden color is due to decreasing frequency of light that is why it partially absorbs violet light while other colors are reflected hence it appears yellowish or golden in color. Caesium atomic number is 55 and its atomic mass is 132.90 g/mol.
Chemical Characteristics
Caesium Atomic Number

Caesium is very reactive and pyrophoric (ignites spontaneously in air). It reacts with water vigorously and explosively, even at low temperatures. Caesium resembles rubidium in its chemical characteristics [3]. Caesium can be handled only under an inert gas, such as argon. It is exit in +1 oxidation state. In compounds it is present as Cs+ and binds ionically with anions. Its salts are usually colorless unless anion itself is colored. Double salts are less soluble but others like phosphate, acetate, carbonate are water soluble. Salts are hygroscopic in nature (attracting and holding water from surrounding). It is difficult to handle Caesium because it reacts spontaneously and react with air. Caesium burns readily to form superoxide. Caesium hydroxides is a strong base which can attack glass. It react with halogens and forms fluoride, chloride, bromide and iodide.
Significance and Uses
What Is Caesium Atomic Number
- Caesium is used in vacuum tubes as a “getter” to clean the traces of oxygen and other gases when sealed.
- Caesium compounds with chlorides are used in photoelectric cells.
- Caesium is used in industries as a catalyst promoter.
- Caesium nitrate is used to make optical glasses.
- Caesium is widely used in the production of electricity, electronics and in chemistry.
- Caesium is also used in the manufacturing of infrared lamps.
- Caesium is widely used in clocks. And it also used to determine the fundamental unit of time.
- Caesium salts are used in water treatment and making mineral water.
- Caesium vapor is used in making magnetometers.
- Caesium is used in molecular biology for density gradient ultracentrifugation.
- It is used in the catalytic conversion of sulfur dioxide into sulfur trioxide.
- Caesiums-137 is radioactive isotope used as a gamma –emitter in various industrial application. It is used in medical field to treat various types of cancer.
- Caesium and rubidium are used as a carbonate to manufacture high quality glass because they reduce electrical conductivity and improve stability.
- It is used as Caesium formate (anion derived from formic acid) for drilling fluids.

Health Hazards
Due to its high reactivity, Caesium is considered as a hazardous metal. People who work in nuclear power station may be exposed to Caesium, otherwise there is no health risk associated with Caesium. People can experience cell damage due to its radiation effects and may suffer from nausea, vomiting, diarrhea and bleeding.
Isotopes of Caesium
It has thirty-nine known isotopes, having atomic masses ranging from 112 to 151. The only stable isotope of Caesium is 133 Cs. Cs-135 has very long half-life of about 2.3 million years.
REFERENCES
[1]. https://www.britannica.com/science/Caesium
[2]. Turekian, K. K.; Wedepohl, K. H. (1961). “Distribution of the elements in some major units of the Earth’s crust”. Geological Society of America Bulletin. 72(2): 175–192.
[3]. Greenwood, N. N.; Earnshaw, A. (1984). Chemistry of the Elements. Oxford, UK: Pergamon Press. ISBN 978-0-08-022057-4.
Other Periodic Table Elements
- Francium
Francium was discovered in 1939. It is very unstable alkali metal and considered the second…
- Tennessine
Tennessine is a synthetic element that was discovered in 2010. It is highly radioactive and…
- Bohrium
Bohrium was discovered in 1976 in Russia. It is radioactive and an artificially prepared element.…
Atomic Mass and Nuclear Binding Energy for Cs-117 (Caesium)
Abstract
This document is part of the Supplement containing the complete sets of data of Subvolume B `Nuclei with Z = 55 - 100' of Volume 22 `Nuclear Binding Energies and Atomic Masses' of Landolt-Börnstein - Group I `Elementary Particles, Nuclei and Atoms', and additionally including data for nuclei with Z = 101 - 130. It provides atomic mass, mass excess, nuclear binding energy, nucleon separation energies, Q-values, and nucleon residual interaction parameters for atomic nuclei of the isotope Cs-117 (Caesium, atomic number Z = 55, mass number A = 117).
- atomic mass;
- mass excess;
- nuclear binding energy;
- nucleon separation energy;
- Q-value;
- nucleon residual interaction parameter
