Isotopic Mass
Description
[ analyzesa peptide sequence and returns a matrix containing the expected massdistribution; a structure containing the monoisotopic mass, averagemass, most abundant mass, nominal mass, and empirical formula; anda matrix containing the expected density function. MD, Info, DF]= isotopicdist(SeqAA)
[ analyzesa compound specified by a numeric vector or matrix. MD, Info, DF]= isotopicdist(Compound)
[ analyzesa compound specified by an empirical chemical formula representedby the structure MD, Info, DF]= isotopicdist(Formula)Formula. The field namesin Formula must be valid element symbolsand are case sensitive. The respective values in Formula arethe number of atoms for each element. Formula canalso be an array of structures that specifies multiple formulas. Thefield names can be in any order within a structure. However, if thereare multiple structures, the order must be the same in each.
isotopicdist(..., ' calls PropertyName', PropertyValue,...)isotopicdist with optionalproperties that use property name/property value pairs. You can specifyone or more properties in any order. Enclose each PropertyName insingle quotation marks. Each PropertyName iscase insensitive. These property name/property value pairs are asfollows:
Find out how isotopes can be detected using mass spectrometry. Learn about isotopes and how they relate to the average atomic mass of an element. If you're seeing this message, it means we're having trouble loading external resources on our website. Isotope ratio mass spectrometer (Isoprime, Micromass, UK). Atom fraction is the amount of a specified atom (isotope) of a chemical element divided by the total amount of.
isotopicdist(..., 'NTerminal', modifies the N-terminal of the peptide.NTerminalValue,...)
isotopicdist(..., 'CTerminal', modifies the C-terminal of the peptide.CTerminalValue,...)
isotopicdist(..., 'Resolution', specifies the approximate resolution of the instrument,given as the Gaussian width (in daltons) at full width at half height(FWHH).ResolutionValue,...)
isotopicdist(..., 'FFTResolution', specifies the number of data points per dalton, tocompute the fast Fourier transform (FFT) algorithm.FFTResolutionValue,...)
isotopicdist(..., 'FFTRange', specifies the absolute range (window size) in daltonsfor the FFT algorithm and output density function.FFTRangeValue,...)
isotopicdist(..., 'FFTLocation', specifies the location of the FFT range (window)defined by FFTLocationValue,...)FFTRangeValue. It specifiesthis location by setting the location of the lower limit of the range,relative to the location of the monoisotopic peak, which is computedby isotopicdist.
isotopicdist(..., 'NoiseThreshold', removes points in the mass distribution that aresmaller than NoiseThresholdValue,...)1/ timesthe most abundant mass.NoiseThresholdValue
isotopicdist(..., 'ShowPlot', controls the display of a plot of the mass distribution.ShowPlotValue,...)
The periodic table only tells us a weighted average of the atomic masses of the different isotopes for an element. For example, if we take a weighted average for the isotopes of Carbon, we get an average atomic mass of 12.011 amu. Like Carbon, many elements exist in nature as a mixture of isotopes.
Isotopic Mass Distribution
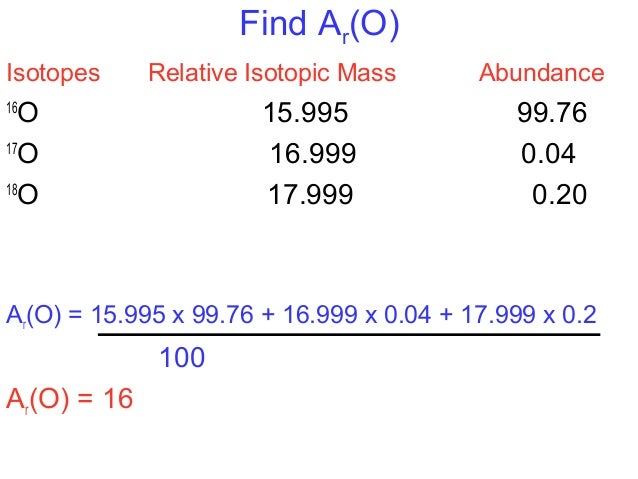
To find the average atomic mass of the element Carbon, we multiply the mass of each isotope by its percent abundance expressed as a decimal. The table below shows the exact mass of each isotope (isotopic mass) and the percent abundance (sometimes called fractional abundance) for the primary isotopes of Carbon.
| Isotope | Exact Weight (Isotopic Mass) | Percentage Abundance |
| Carbon-12 | 12.0000 | 98.90 % |
| Carbon-13 | 13.0033 | 1.10 % |
To find the average atomic mass for Carbon:
Average Atomic Mass = (12.0000)(.9890) + (13.0033)(0.0110) = 12.011 amu
The answer, 12.011 amu, is the same value found for Carbon on the periodic table.
Exercise: Calculate the average atomic mass of Nitrogen (N) based on the information given.

| Mass Number | Exact Weight (Isotopic Mass) | Percentage Abundance |
| 14 | 14.003074 | 99.63% |
| 15 | 15.000108 | 0.37% |
The exact mass of an isotope is something that is determined experimentally. To find the average atomic mass experimentally a mass spectrometer is used. This laboratory instrument works based on the differences in the mass of the different isotopes of an element. Because isotopes for an element have different masses, they can be separated, and the percentage of each isotope measured. From these measurements the average atomic mass can be calculated.
Isotopic Mass Spectrum
Review of Important Terms:
Isotopic Mass Calculator
- Mass Number is the number of protons and neutrons in an isotope. It is a whole number. We use mass number in naming isotopes, like Carbon-12 or Oxygen-17.
- Atomic Mass is the mass of the entire atom of an isotope. This includes electrons as well as a slight change in mass due to binding energy.
- Average atomic mass is the weighted average of the isotopes for an element. This is what is listed below the element symbol on the Periodic Table.
Exercise: Use the simulation to compare the average atomic mass and determine why Hydrogen (1.0079) is close to a whole number but Chlorine (35.453) is not. In the PhET simulation, click on 'Mixtures', and at the bottom of the page select 'Nature's Mix'. On the periodic table provided, select H or Cl. |
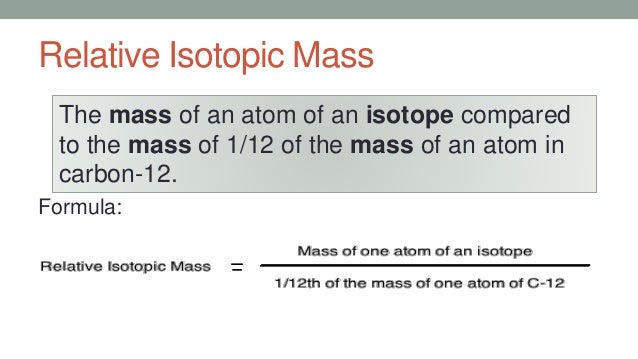
Definition Of Relative Isotopic Mass
