Uranium Mass Number
Atomic Mass of Uranium Atomic mass of Uranium is 238.0289 u. Uranium is of great importance as a nuclear fuel. Adobe acrobat pro for mac download. Nuclear fuels are used to generate electrical power, to make isotopes, and to make weapons. Much of the internal heat of the earth is thought to be due to the presence of uranium and thorium. Uranium-238, with a half-life of 4.51 x 10 9 years, is used to estimate the age of.

Although two to three neutrons are produced for every fission, not all of these neutrons are available for continuing the fission reaction. If the conditions are such that the neutrons are lost at a faster rate than they are formed by fission, the chain reaction will not be self-sustaining.
At the point where the chain reaction can become self-sustaining, this is referred to as critical mass.
In an atomic bomb, a mass of fissile material greater than the critical mass must be assembled instantaneously and held together for about a millionth of a second to permit the chain reaction to propagate before the bomb explodes
Uranium Atomic Number And Mass Number
The amount of a fissionable material's critical mass depends on several factors; the shape of the material, its composition and density, and the level of purity.
A sphere has the minimum possible surface area for a given mass, and hence minimizes the leakage of neutrons. By surrounding the fissionable material with a suitable neutron 'reflector', the loss of neutrons can reduced and the critical mass can be reduced.
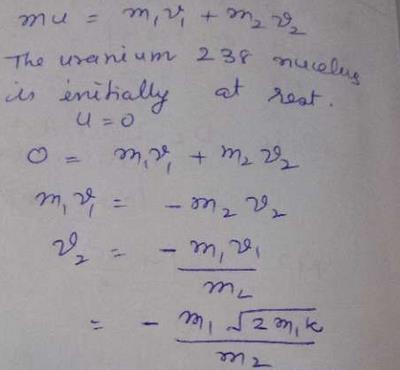
Uranium-239 Mass Number
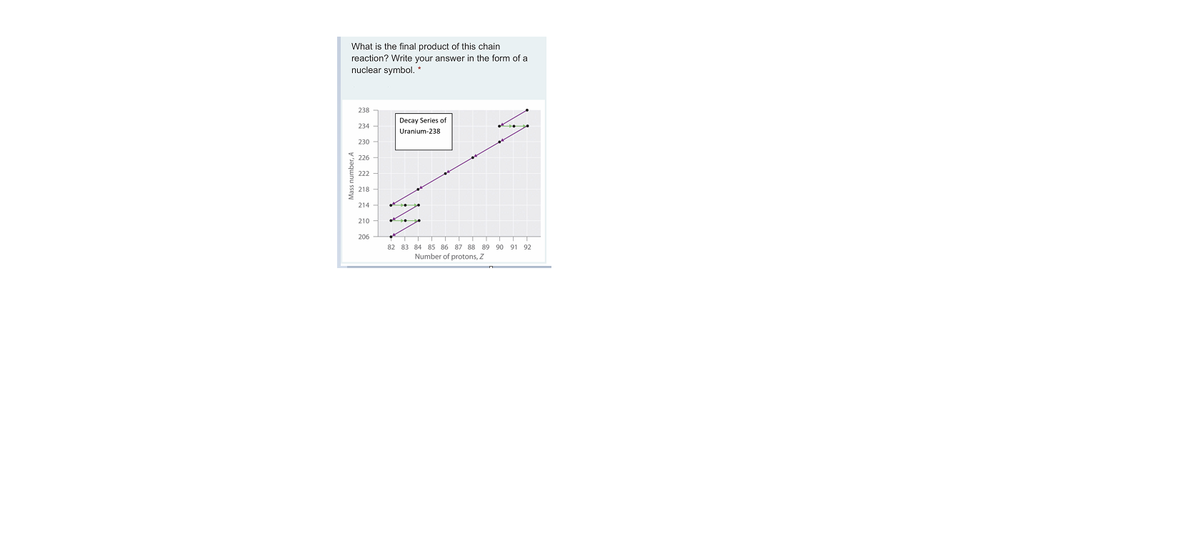
Number Of Neutrons In Uranium
By using a neutron reflector, only about 11 pounds (5 kilograms) of nearly pure or weapon's grade plutonium 239 or about 33 pounds (15 kilograms) uranium 235 is needed to achieve critical mass.
